Many long chain alcohols exhibit some surfactant properties. Prominent among these are the fatty alcohols, cetyl alcohol, stearyl alcohol, and cetostearyl alcohol (consisting predominantly of cetyl and stearyl alcohols), and oleyl alcohol. In order to further increase these properties, fatty alcohols are ethoxylated so that they become more hydrophilic.
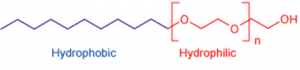
Absorption and hence surface active properties are high when the solubility is poor, e.g. when the ratio of the hydrophylic part is low. However practical formulations need good solubility and therefore are a compromise between surface active properties and solubility.
The ratio of the hydrophilic part to the hydrophobic part is expressed in the so called HLB value (Hydrophylic Lipophilic Balance). This value can be calculated by dividing the molecular mass of the hydrophilic part of the molecule by the total molecular weight of the molecule. The resulting number is then divided by 5 so that the HLB value can range from 0 to 20, where a value of 0 corresponds to a completely lipophilic/hydrophobic molecule, and a value of 20 corresponds to a completely hydrophilic/lipophobic molecule.
The HLB value can be used to predict the surfactant properties of a molecule. In wax emulsions the HLB value of the surfactants ranges from 10 to 16.